- This event has passed.
Sixth Philosophy of Cancer Biology Workshop
4 March | 9 h 30 min - 6 March | 13 h 00 min

The Sixth Philosophy of Cancer Biology Workshop was held in Bordeaux, France, from March 4th to 6th, 2024.
Cancer is one of the main causes of death globally according to the World Health Organization. The biological complexity and heterogeneity of this disease (or group of diseases) make it very difficult to apprehend, control, and cure. We strongly believe that more theoretical and conceptual efforts can benefit cancer research. To this aim we have initiated a series of international workshops whose aim is to gather philosophers, scientists, and medical doctors to discuss theoretical and conceptual challenges that are central to cancer research.
This edition will mostly focus on two topics: (1) the role of the microbiota in cancer development and dissemination, a question that attracts increasing attention but remains challenging to address, (2) metastasis, which is considered as a major challenge for cancer treatment and a leading cause of cancer death, and for which innovative conceptual and theoretical frameworks are needed. The workshop, especially during Day 3, will explore many other emerging conceptual and theoretical issues in cancer research.
**
Invited speakers:
- Ilaria Elia (KU Leuven, Belgium)
- Andrew Ewald (Johns Hopkins, Baltimore, USA)
- Peter Friedl (Radboud University Nijmegen, Netherland)
- Lucie Laplane (IHPST & Gustave Roussy, Paris, France)
- Cristina Lo Celso (Imperial College London, UK) (Canceled)
- Carlo Maley (Arizona State University, USA)
- Guillaume Montagnac (Gustave Roussy, France)
- Julie Pannequin (Institut de Génomique Fonctionnelle, Montpellier, France)
- Maria Rescigno (Humanitas University, Italia)
- Gregory Sepich-Poore (UCSD, USA)
- Eric Solary (Gustave Roussy, Paris, France)
- Orsolya Vincze (ImmunoConcEpT, CNRS Bordeaux & La Rochelle University, France)
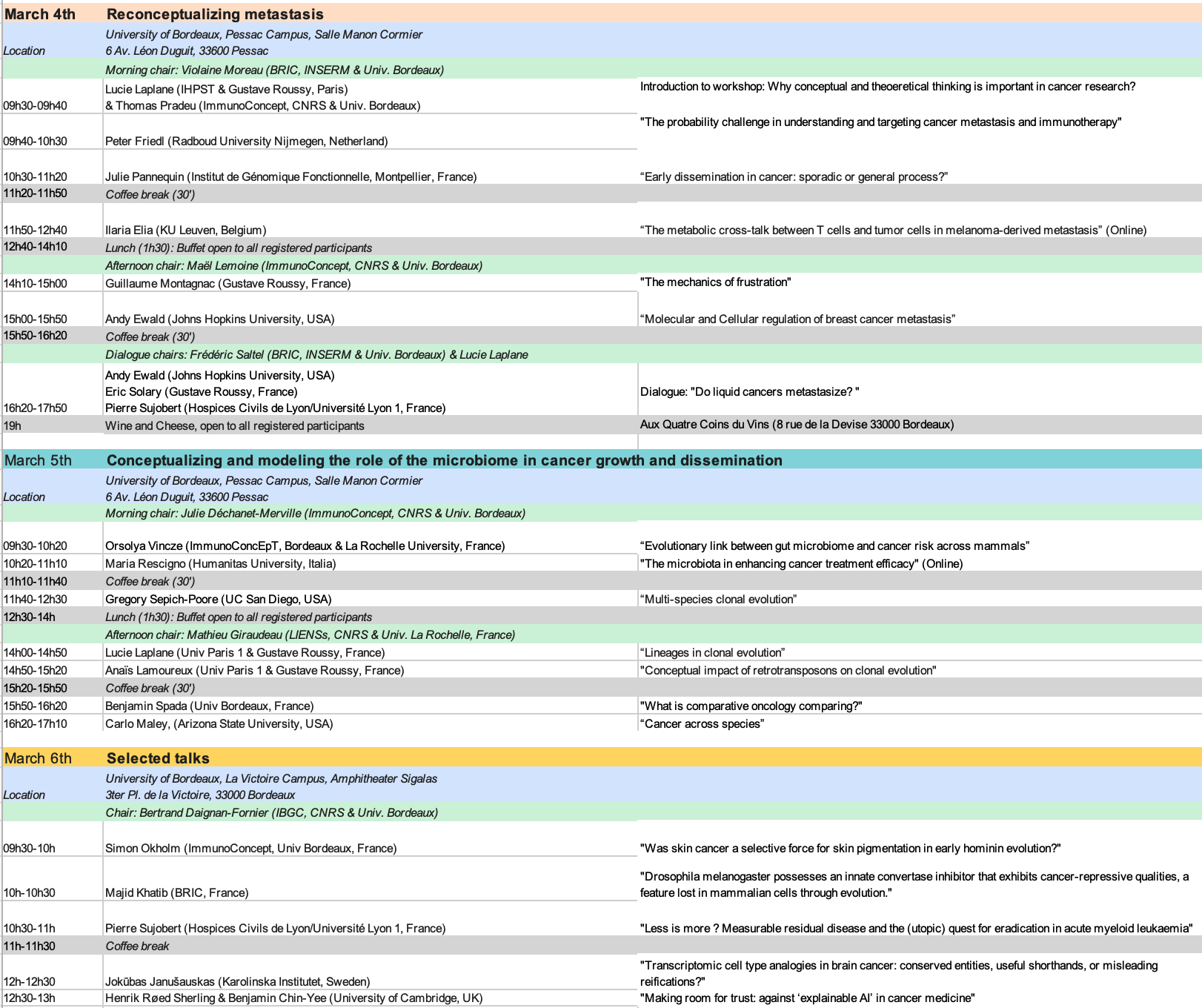
Program
Abstracts
Ilaria Elia (KU Leuven, Belgium), The metabolic cross-talk between T cells and tumor cells in melanoma-derived metastasis
Activation of the endogenous immune system through immunotherapy has greatly improved cancer treatment. Drugs that induce T cell function lead to the specific targeting and killing of tumor cells. Yet, especially in metastatic sites, a significant subset of tumors evade the immune system. Indeed, the tumor microenvironment (TME) represents a hostile niche that inhibits the function of infiltrating T cells and transforms them into an exhausted state. Recently, metabolic reprogramming has emerged as a key hallmark of immune responses. To support their proliferation and survival, T cells use fuels to generate precursors required for macromolecular synthesis, energy, and pro-survival pathways. As hyperproliferative cancer cells possess an overactive metabolism, depletion of nutrients and accumulation of metabolic waste products might represent a major nutritional hurdle for infiltrating T cells. Our goal is to map the metabolic requirements of T cells within the TME, develop strategies to potentiate T cell metabolism within this hostile niche, and thus use metabolic therapy to reactivate exhausted T cells and drive their cytotoxic function in immunocompromised metastasis.
Andrew J. Ewald, Ph.D. (DeAcetis Professor and Director, Department of Cell Biology, Director, Giovanis Institute for Translational Cell Biology, Johns Hopkins University, School of Medicine, Baltimore, MD, 21228, USA), Intrinsic defenses against cancer invasion and metastasis
Cancer mortality is driven by metastasis, the process by which cells escape from the primary tumor and colonize distant organs. We have shown that luminal breast cancer cells can initiate invasion by expressing basal epithelial genes, such as keratin 14 (K14). K14+ luminal breast cancer cells collectively invade and intravasate as adherent clusters. Conversely, triple negative breast cancer (TNBC) cells invade and metastasize in a hybrid EMT state, expressing both E-cadherin and vimentin, with collective spread also favored. In both subtypes, we observe transitions in cell state as the cancer cells expand to form growing metastases in the distant organ. We next sought to understand how intercellular interactions regulated metastasis through deletion of key proteins in the adherens junction (E-cadherin) and tight junction (claudin 7). We demonstrated that E-cadherin acts as an invasion suppressor and a metastasis promoter- when deleted cells invaded more but rapidly died due to anoikis. In preliminary experiments, claudin 7 acts as an invasion suppressor and a metastasis suppressor- when we delete claudin 7 we see more invasion in culture, equivalent cell survival, and increased colony formation. We are currently working to develop a theoretical framework that could help explain why cancer cells preferentially metastasize in groups and connect their cell survival selectively to the expression of junctional proteins, such as E-cadherin. We are working with Thomas Pradeu and Bertrand Daignan Fornier to evaluate whether concepts from the evolution of multicellularity could inform our understanding of the collective strategies driving metastasis.
Peter Friedl (Dept. of Medical BioSciences, Radboudumc, Nijmegen, The Netherlands), The probability challenge in understanding and targeting cancer metastasis and immunotherapy
Textbooks and review articles present principles of cancer biology in multi-color cartoons pointing out associations and cause-consequence cellular and molecular programs, which may determine cell and tissue function. In cancer metastasis, molecular drivers have been identified, which result in cancer cell migration, dissemination through the body to enable dissemination and seeding. Molecular programs further support survival strategies which enable the cells to survive critical challenge, such as molecular-targeted and immunotherapy. These mechanisms are typically presented and discussed as deterministic events, which can be modified by targeted therapy. However, recent quantitative biology and in silico modeling as well as clinical experience indicate these biological processes as probabilistic programs, which are based on chains of shallow or bistable effects in structural and molecular networks and mechanisms of signaling compensation or even signaling failure under particular circumstances. I will discuss experimental wet-lab results from cancer metastasis and immunotherapy research which are based on chains of probabilistic events, which mandate a spectral approach to understand and present the probabilistic nature of fundamental and applied cancer research. Appreciating chains of probabilities as fundamental principle in life science will allow to improve strategies of designing experiments and analysis approaches as well as basic and academic teaching methods.
Jokūbas Janušauskas (doctoral student, Karolinska Institutet), Transcriptomic cell type analogies in brain cancer: conserved entities, useful shorthands, or misleading reifications?
Cancer cells are oftentimes compared to fetal and mature non-pathological cell types. Early comparisons of this kind were mainly based on morphological resemblance and aimed at broadly systematizing the diversity of neoplastic cells. In recent years, however, following the advent of single-cell analytical methods, cell type identities have been increasingly defined anew in transcriptomic terms. This has indeed helped to characterize and systematize the cellular diversity of both healthy and cancerous tissues in unprecedented detail, making the repertoire of recognized cell types more nuanced and, above all, vast. Moreover, it has changed the very basis of cell type comparisons: cell type similarity can now be assessed “quantitatively” based on gene expression. Yet, instead of clarifying and consolidating unique cancer-to-healthy correspondences in the newfound plethora of cell types, the transcriptomic comparison approach has, for the most part, merely exposed uncertainties concerning the concepts of cell types and states and the blurry borders within these categories. This ultimately raises into question the validity and value of extensive commitment to such cell type analogies.
The problem is particularly rampant in single-cell transcriptomics studies of glioblastoma, a notoriously heterogeneous kind of tumor arising in the already-complex brain tissue. In this talk, I will: 1) provide an overview of the current field consensus and our own ongoing efforts in classifying glioblastoma cells through developmental or physiological counterparts, highlighting the methodological and conceptual shortcomings therein; 2) argue against the use of exclusively transcriptomics-based analogies for cell type identities, advocating instead for an increased emphasis on spatial context and role in tissue architecture.
Majid Khatib (BRIC INSERM) : Drosophila melanogaster possesses an innate convertase inhibitor that exhibits cancer-repressive qualities, a feature lost in mammalian cells through evolution.
The kexin-like proprotein convertases (PCs) play a pivotal role in health and disease by orchestrating the cleavage and maturation of diverse protein precursors. These enzymes selectively activate protein precursors that display specific consensus sequences. The conversion of PC substrates involves the collaboration of seven family members: PC, PC2, Furin, PC4, PC5, PACE4, and PC7. Our prior research has been instrumental in exploring the implications of PCs in cancer and, more recently, in the context of anti-tumoral immune responses. Despite the widespread distribution and evolutionary conservation of PCs within the secretory pathway, the identification of endogenous inhibitors or mechanisms regulating their enzymatic functions in human and murine cells has remained elusive. However, our investigation in Drosophila melanogaster has unveiled an endogenous PCs inhibitor with the ability to effectively inhibit all members of the mammalian PC family in vitro. This inhibitor has shown promise in mitigating the malignant phenotype of mammalian cancer cells and suppressing tumor growth in vivo. These results indicate a possible depletion of PC regulators over the course of evolution, potentially playing a role in the initiation of cancer in mammals. Building upon these findings, I will explore how insights from research on non-human entities can provide valuable perspectives for applications in human health and enhance our understanding of cancer. This will involve posing inquiries that would benefit from philosophical scrutiny that allow the development and testing of philosophical questions of interest linked to evolution and cancer.
Anaïs Lamoureux (Univ Paris 1 & Gustave Roussy) : Conceptual impact of retrotransposons on clonal evolution
The clonal evolution model provides a framework for understanding the progression of cancer cells. According to this model, cancer cells accumulate genetic mutations over time, and these mutations are passed down to their descendants, leading to genetic diversity within the tumor. Some of those mutations confer selective advantages, causing certain cell populations (clones) to dominate and expand. However, this model is rooted in certain conceptual assumptions, which we aim to challenge by exploring the involvement of retrotransposons in cancer initiation and progression. In recent years, it has become evident that transposable elements, particularly retrotransposons, play a significant role in driving cancer transformation and progression. However, their inclusion in the clonal evolution model remains unexplored. To incorporate retrotransposons into this model, theoretical adjustments must be made, such as reevaluating the concept of fitness, considering alternatives to bifurcation as the sole evolutionary pathway, and redefining our understanding of lineages.
Lucie Laplane (Université Paris I-Panthéon Sorbonne) Lineages in clonal evolution
Tumors are made up of heterogeneous cells. This heterogeneity makes cancer cells difficult to target and contributes to therapeutic avoidance and relapses. The clonal evolution model describes the dynamical processes of emergence, growth, decline or disappearance of clones constituting a tumor in space and time. In this model, lineages play an important role as they support the transmission of intrinsic cancer cells properties contributing to their fitness. However, there are several short-comings in the epistemology of cancer lineages. First, lineages are considered as inviolable. I will show several independent lines of data indicating that they are not. Second, the clonal evolution model has focused on long-term inheritance, tracked by mutations and stable epigenetic traits. Yet, short-term inheritance might be as relevant to explain clonal dynamics, especially in the context of metastasis and treatment. I will explore some solutions to revise our conception of cancer cell lineages that can accommodate the empirical evidence, and then derive from these solutions some potential avenues for new therapeutic strategies.
(Canceled) Cristina Lo Celso (Imperial College London, UK), Lessons learned from looking at healthy and malignant haematopoietic cells in the bone marrow
Leukaemia is known as cancer of the blood, however it develops and grows in the bone marrow, the tissue where blood stem cells maintain the production of billions of blood cells every day. Deeply encased inside hard bones, and formed of the softest parenchyma within the body, the bone marrow has been traditionally inaccessible to direct observation. I used intravital microscopy to gain the first information on the dynamic interactions between healthy blood stem cells and leukaemia cells and the bone marrow microenvironment. More recently, whole bone preparations, multispectral immunofluorescence and microscopy and AI image analysis methods are allowing us to grasp the complex organization of blood precursor cells in the bone marrow, which has often been referred to as a ‘liquid’ tissue. With genomics analyses indicating that clonal haematopoiesis is the likely precursor of leukaemia, microscopy analyses may provide some clues on the mechanisms allowing clonal haematopoiesis to develop. Mathematical modelling aids interpretation of microscopy datasets and their integration with flow cytometry and genomics datasets, providing new tools to understand the links between blood progenitor cells’ unexpected migratory behaviour, bone marrow tissue organization, and healthy and malignant haematopoiesis.
Simon Okholm (University of Bordeaux): Was skin cancer a selective force for skin pigmentation in early hominin evolution?
The MC1R gene, one of several responsible for constitutive dark skin pigmentation, underwent fixation approximately 1.2 million years ago among savannah-dwelling hominins in response to ultraviolet radiation from the sun.1 While various theories have been offered for skin pigmentation as an adaptive trait in early hominin evolution, such as protection from folate photolysis to limit fatal birth defects2 or enhanced skin barrier protection against infections3, the idea that pigmentation evolved to safeguard against skin cancers was long dismissed. Skin cancers result from modern human migrations, evolutionary mismatch, and occur after peak reproductive age.4,2,3 However, the skin cancer hypothesis has recently been revived.5,6
Here, we address the revival of the skin cancer hypothesis as a selective pressure in the evolution of skin pigmentation, using tools from philosophy7 in a three-step process to evaluate its plausibility. The first part is clarificatory, where we sketch the controversy and elaborate on the old arguments and evidence for and against it. In the second part, we identify three new arguments – related to the longevity of hunter-gatherers, the inclusive fitness behavior of post-reproductive individuals, and albinism as a model for hominins’ skin cancer susceptibility – and scrutinize each for its added value. When comparing old with new arguments, we find that most remain either unsubstantiated or supported by circumstantial evidence. This prompts us to question whether we “read adaptive value into almost any aspect of an organism,” given our incomplete understanding of hominins and their ancestral environment, echoing Blum’s caution from half a century ago4. In the third part, we appraise Blum’s caution by asking and reflecting on what evidence would be instrumental for testing the skin cancer hypothesis to advance the debate on what drove skin pigmentation in early hominin evolution.
References
1.Rogers, A. R., Iltis, D. & Wooding, S. Genetic Variation at the MC1R Locus and the Time since Loss of Human Body Hair. Curr. Anthropol. 45, 105–108 (2004).
2.Jablonski, N. G. & Chaplin, G. Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. 107, 8962–8968 (2010).
3.Elias, P. M. & Williams, M. L. Re-appraisal of current theories for the development and loss of epidermal pigmentation in hominins and modern humans. J. Hum. Evol. 64, 687–692 (2013).
4.Blum, H. F. Does the Melanin Pigment of Human Skin Have Adaptive Value?: An Essay in Human Ecology and the Evolution of Race. Q. Rev. Biol. 36, 50–63 (1961).
5.Greaves, M. Was skin cancer a selective force for black pigmentation in early hominin evolution? Proc. R. Soc. B Biol. Sci. 281, 20132955 (2014).
6.Osborne, D. L. & Hames, R. A life history perspective on skin cancer and the evolution of skin pigmentation. Am. J. Phys. Anthropol. 153, 1–8 (2014).
7.Laplane, L. et al. Opinion: Why science needs philosophy. Proc. Natl. Acad. Sci. 116, 3948–3952 (2019).
Carlo Maley (Arizona State University, USA), Cancer across species
Cancer is a problem for all multicellular species. In fact, cancer was probably one of the barriers to the evolution of multicellularity. Since multicellularity evolved ~2 billion years ago, cancer has been one of the selective pressures on organisms, leading to the evolution of a variety of cancer suppression mechanisms. Despite the ubiquity of cancer, very little is known about cancer in non-human species. The growing field of comparative oncology has been gathering data on cancer across species. We are trying to answer two fundamental questions. First, what explains any patterns of cancer resistance/susceptibility across species? Second, what mechanisms has evolution discovered for the suppression of cancer that we might translate to cancer prevention in humans? I will review the answers that are emerging to both of those questions.
Julie Pannequin (Institut de Génomique Fonctionnelle, Montpellier, France), Early dissemination in cancer: spordoc or general process?
The ability of yet benign tumor cells to disseminate from the primary tumor before transforming to their full metastatic potential has recently received increasing attention. So far, for intestinal tumorigenesis, the existence of such function of cells at early benign stages could not be demonstrated. Here, we introduced and characterized a genetic mouse model with specific tamoxifen-driven induction of tumorigenesis in intestinal tdTomato-tagged cells. This model enabled us, already at the adenoma stage, to detect intestinal early disseminated tumor cells (eDTCs) in the liver. This early dissemination was concomitant with elevation of TIMP-1 plasma level and correlated with substantial hepatic infiltration of myeloid cells including macrophages and neutrophils. Myeloid cells enrichment facilitated metastatic growth in this organ when challenged with carcinoma cell inoculation.
Importantly, we observed elevated TIMP-1 levels and presence of eDTCs in the blood of patients with intestinal polyps, highlighting the clinical relevance of this study.
Altogether, this study revealed that intestinal eDTCs play a crucial role in the formation of a hepatic metastatic niche by their ability to secrete TIMP-1 leading to recruitment of myeloid cells to the liver. This functionally includes the role of eDTCs in the concept of pre-metastatic niche formation.
Based on this study, I will propose a discussion on whether every single (solid) tumor disseminates at very early stages.
Maria Rescigno (Humanitas University, Milan Italy), The microbiota in enhancing cancer treatment efficacy
There is no doubt that immunotherapy, parcularly Immune checkpoint blockade (ICB), has drascally improved treatment of metastac cancer paents. Microbiota composion has been proposed to be one of the reasons for failure or success. ICB works via the acvaon or reacvaon of T cells that are ‘switched off’ by tumor cells or by the tumor microenvironment. Even advanced metastac disease, previously considered as untreatable, can benefit from cancer immunotherapy. However, sll a good proporon of paents does not respond to therapy or acquires resistanceduring treatment. Some genera or species of bacteria have been associated with treatment response or toxicity, but as the composion of the microbiota is not stac, rather, it is very dynamic there is promise that by changing the microbiota composion, or by harnessing the microbiota ‘secrete’ tricks, one can improve treatment efficacy or reduce toxicity. Several players, including diet, prebiocs, probiocs and postbiocs have been proposed to shape the microbiota. Here we will discuss how to train the microbiota to increase ICB efficacy and to reduce toxicity.
Henrik Røed Sherling & Benjamin Chin-Yee (University of Cambridge): Making room for trust: against ‘explainable AI’ in cancer medicine
Can we trust AI to diagnose cancer? Growing research focuses on applying AI to aid in diagnosis, prognosis, and treatment of cancer. For example, AI has been used to diagnose genetic subtypes of acute leukemia using digitized images of peripheral blood (Hehr et al. 2023). Use of AI for such purposes raises concerns over its ‘black box’ nature, motivating attempts to render the AI ‘explainable’—to enable understanding of how the algorithm arrived at its output in order to justify reliance. Explainability seems to be a key desideratum for AI, especially when stakes are high as in cancer medicine. Achieving ‘explainable AI’ is thus seen as necessary to respond to the above question: in order to trust AI, it must be explainable. Lack of explainability, or ‘essential epistemic opacity’, it is argued, undermines trust.
This paper, an interdisciplinary collaboration between a philosopher and a practicing hematologist, argues that this question, though commonly raised in literature on medical AI, is malposed, and the response it engenders is misguided. We reject two common assumptions in this literature.
First, we reject that AI is essentially epistemically opaque in a way that is relevant to scientific and medical practice. Instead, we argue that teleological explanations, which refer to what an AI was designed to do in a given training context, combined with evidence of reliability and similarity of context, are often sufficient to justify reliance. We illustrate this point by analogy with other ‘black box’ medical interventions.
Second, we reject the commonly assumed link between explainability and trust, which sees explainability as a path to establishing trust. We argue that, though explainability supports justified reliance, it is in fact orthogonal to trust: when one is justified in relying, questions of trust do not arise.
By rejecting this second assumption, our account makes room for trust where trust is needed. Trusting use of AI in cancer medicine means trusting the researchers, institutions, and practitioners responsible for validating and implementing such tools. Trust is required for the cooperative processes of scientific research, clinical evaluation, and critical appraisal that allow for reliable use of AI in practice, no different than other diagnostic and therapeutic technologies in cancer medicine. In short, there is no novel problem of trust in AI in cancer medicine.
We support our argument with real-world examples of AI developments in cancer medicine, specifically for diagnosis and prognosis in hematologic cancers. By clarifying the relationship between explainability, justified reliance, and trust, our paper has critical upshots for medical and scientific practice, articulating the kinds of explanations and evidence required for justified use of AI while refocusing questions of trust to where they actually matter. Overall, our argument supports careful development of AI in cancer medicine while emphasizing the need for ongoing rigorous clinical validation. In this way, we provide philosophical support for perspectives recently expressed by scientists and physicians (e.g., Ghassemi et al. 2021), who argue for greater attention to clinical validation of AI over misguided pursuit of explainability as a basis for trust.
Benjamin Spada (Univ Bordeaux): What is comparative oncology comparing ?
Comparative oncology is an heterogeneous field which studies cancer in non-human organisms. One can already find evidence of such comparative approaches about cancer in the beginning of the 20th century1,2. However, the field has known a regain of activity in the last fifteen years. With the development of new tools and methods as well as an attempt to define the research agenda of the field3,4, comparative oncology is on a rising curve. Yet, even though the field is producing more and more data on cancer across species5,6, it struggles to find legitimacy in the eyes of mainstream oncology. In fact, for many biologists, it is not obvious why there would be any interest in studying cancer outside of the human case. To gain this interest, the field must first be clear on its goals, its promises it and how they will be fulfilled. We argue that it isn’t the case for now because of the novelty of the field and the resulting lack of conceptual grounding. We argue that there are some mismatch between the goals and the methods which might be resolved by a conceptual work on the very core of the field: comparatism.
In fact, we believe that one way to be clearer about the very activity of comparative oncology is to explain what exactly is being compared. For it is not obvious what is or should be compared and why. We believe and will argue that this lack of methodological and conceptual clarity might act as a brake for the development of the field. For this we will produce a taxonomy of the different types of comparatisms that are gathered under the label “comparative oncology “ and address their specificities and the challenges they each have to face. Finally we will provide criteria to sort out which comparatism is useful for which goals of comparative oncology. The result should be a clearer and conceptually well-grounded analysis of the the types of comparatism in comparative oncology.
References:
1. Smith, E. F. Further Evidence That Crown Gall of Plants Is Cancer. Science 43, 871–889 (1916).
2. Smith, E. F. Cancer in Plants and in Man. Science 61, 419–420 (1925).
3. Nunney, L., Maley, C. C., Breen, M., Hochberg, M. E. & Schiffman, J. D. Peto’s paradox and the promise of comparative oncology. Philos. Trans. R. Soc. B Biol. Sci. 370, 20140177 (2015).
4. Kokko, H., Schindler, S. & Sprouffske, K. Chapter 22 – Searching for a Cancer-Proof Organism: It’s the Journey That Teaches You About the Destination. in Ecology and Evolution of Cancer (eds. Ujvari, B., Roche, B. & Thomas, F.) 247–254 (Academic Press, 2017). doi:10.1016/B978-0-12-804310-3.00022-3.
5. Vincze, O. et al. Cancer risk across mammals. Nature 601, 263–267 (2022).
6. Compton, Z. T. et al. Cancer Prevalence Across Vertebrates. 2023.02.15.527881 Preprint at https://doi.org/10.1101/2023.02.15.527881 (2023).
Pierre Sujobert (Hospices Civils de Lyon/Université Lyon 1) : Less is more ? Measurable residual disease and the (utopic) quest for eradication in acute myeloid leukaemia
Unless solid cancers which can be cured by surgery when diagnosed at localized stages, the only efficient way to treat patients haematological malignancies (HM) is systemic chemotherapy and/or targeted molecules followed by allogeneic hematopoietic stem cell transplantation in high risk patients. Even if most patients achieve a response, only a proportion (depending on the disease) are cured, and prospective identification of these patients is an important goal for clinicians. HM, due to their potential for repetitive sampling, pioneered the concept of minimal (or measurable) residual disease (MRD), which is now generalizable to other cancers with circulating tumour DNA analysis. Using highly sensitive techniques such as molecular biology or flow cytometry, MRD evaluation aims at identifying persisting tumoral cells able to replenish the disease.
Although MRD assessment has proven effective in distinguishing patient outcomes across various hematological malignancies, its direct application to personalize treatments remains confined to a select few, where clinical benefits have been demonstrated. For instance, in acute myeloid leukemia (AML), MRD strategies have not yet shown significant efficiency, although their prospective adaptation could be crucial for this disease. We argue that the limited clinical value of MRD in AML arises from conceptual inconsistencies.
First, MRD is a metonymic concept: instead of detecting residual cancer cells able to drive relapse, MRD detects a molecular abnormality of cancer cells, or an abnormal phenotype. Similarly, cancer diagnosis relies on metonymy: the identification of irregular tissue organization defines the disease. While this approach is effective for diagnosing sick patients, it leads to overdiagnosis in screening campaigns involving healthy individuals. In the case of MRD as in screening campaigns, applying the metonymic definition of cancer to asymptomatic patients poses the same challenge of overdiagnosis. Specific examples, like the persistence of clonal hematopoiesis will be discussed during the meeting.
Secondly, the foundation of MRD in Acute Myeloid Leukemia (AML) rests upon an incomplete understanding. Its objective is to identify a residue capable of fuelling the entire disease—a concept that aligns with the leukaemia stem cell model. However, two key limitations emerge from this approach. Firstly, assessing leukemic stem cells in patients relies on immunophenotyping rather than the true definition based on functional assessments in mice. This discrepancy could contribute to the inefficacy of MRD measurements. The second limitation lies in the exclusive focus on cancer cells, disregarding the microenvironment, which plays a significant role in the potential of residual leukemic cells to trigger disease relapse
Thirdly, MRD’s clinical value assumes that cancer should be entirely eradicated by treatment. However, evolutionary perspectives suggest alternative strategies, like adaptive therapies focusing on tumour control rather than complete eradication. Although applying these strategies to acute leukaemia is debatable, promising results in more indolent cancers have emerged.
Finally, the clinical utility of MRD assessment remains uncertain without highly efficient (and non toxic) treatments. Even with accurate identification of patients prone to relapse, the actual clinical benefit of early treatment intensification is yet to be established.
Considering these limitations, we propose the following strategies for the future of MRD in haematological malignancies: 1/ MRD should be developed primarily in curable diseases for dose-intensity adjustments to reduce toxicity; 2/ the evaluation of relapse risk should focus on functional assessments; and 3/ MRD monitoring should integrate evaluation of the tumour microenvironment.
Orsolya Vincze (ImmunoConcept, Bordeaux & LIENSs, La Rochelle, France), Evolutionary link between gut microbiome and cancer risk across mammals
Cancer is a ubiquitous problem of mammals but its prevalence varies widely from species to species, as well as along the phylogeny. Unraveling the source of cross-species variability in cancer resistance promises novel perspectives at the forefront of cancer research. Human-oriented oncological research has recently become deeply focused on the role of immunity and immune deficiency in shaping cancer predisposition and treatment efficiency. While research is steadily accumulating proof of the critical role of immunity in cancer defense, little is known about how immunity shapes cancer risk across mammals. During my talk, I’ll discuss cross-species variability in cancer risk, as well as the potential role of immunity in shaping this. Most importantly, I will present preliminary findings regarding the role of species-specific intestinal microbiome profiles, a critical element of immune priming and development, in shaping mammalian cancer risk. Our analyses cover both overall microbial diversity, as well as the prevalence of specific bacterial lineages in shaping cancer risk across taxa. I will present our results in parallel with similar explorations using humans and laboratory model organisms while considering both the direct and indirect effects of microbes on oncogenesis and defense.
Canceled: Marc Billaud, Thibaut Serviant-Fine, Arnaud Vigneron (Centre de Recherche en Cancérologie de Lyon ; Centre Léon Bérard, Lyon) : Metabolic reprogramming is a widely used concept describing the bioenergetic changes central to the oncogenic process.
This notion suggests that oncogenes and inactivated tumor suppressors instigate a persistent restructuring of metabolic networks crucial for generating the necessary biomass supporting cancer cell growth. However, recent discoveries indicate that malignant cells possess a capacity for dynamic adaptation to their ever-changing microenvironment, demanding a remarkable level of metabolic flexibility and plasticity. Moreover, their metabolic characteristics are intricately molded by thermodynamic constraints. These emerging findings challenge the deterministic notion of metabolic reprogramming.
The translation of metabolic issues into the language of programming and reprogramming, unconventional concepts in the field of intermediary metabolism, inevitably shapes our comprehension of tumor metabolism and steers the development of research programs. We reassess specific examples of metabolic reprogramming in light of the current data. While acknowledging the valuable insights offered by this concept, we aim to highlight its inherent limitations, urging a redefinition that incorporates enlightening findings from diverse areas of metabolic research. We also explore the hypothesis that metabolic reprogramming served as a boundary concept, bridging the domains of cancer genetics and intermediary metabolism, thereby contributing to the establishment of the field of cancer metabolism.
Canceled without notification: Haley Cionfolo (Rollins School of Public Health at Emory University) & Caleb Hazelwood (Duke University): A Biological and Philosophical Comparison of Early Fetal Tissue and Tumors in the Post-Roe Era
The collapse of Roe v. Wade in June 2022 has had many consequences for reproductive rights in the United States, especially increasing restrictions on early abortions under 12 weeks. A New York Times editorial in early 2023, however, questioned our popular understanding of what is actually removed in an early abortion. In an article entitled “Early Abortion Looks Nothing Like You’ve Been Told”, Erika Bliss published images of isolated aborted fetal tissue from five weeks to nine weeks. These photographs did not show the “mini baby” imagery that is often conjured, but, rather, clumps of undifferentiated tissue.
While this raises many ethical qualms, it also opens the door to different questions related to the epistemology and ontology of biology. What is an early-stage fetus? More specifically, how does the early fetus and its maternal home compare to other foreign bodies hosted within a patient, such as a tumor, and to what extent? What attributes could allow them to be viewed and treated similarly?
In this paper, I contend that early-stage fetuses and tumors are biologically and philosophically similar. Therefore, I will argue that a host has no more moral responsibility to an early-stage fetus than they do a tumor. I will begin with summarizing the opposing position, that fetuses are separate biological and philosophical entities with agency, unlike a tumor, and must be treated as individual patients. I will then refute this claim by defining “early-stage fetus” and “tumor” and describing their biological similarities across different subdisciplines, including biomechanics, developmental biology, symbiosis, immunology, and medicine. In biological terms, I will also describe fetal tissue and tumor uniqueness as exceptions to “inclusive fitness theory” due to their somatic self-interest (Stencel & Suárez, 2021).
Moreover, I will synthesize existing arguments regarding the lack of philosophical and historical separation between foreign body and host. This evidence will demonstrate that, similar to a tumor, fetal tissue cannot be treated as its own entity relative to the host, refuting the popular “container theory” and rhetoric of the “fetal patient” (Kukla & Wayne, 2018; Lyerly et al., 2008; Nuño de la Rosa et al., 2021). I will also explain why this does not actually contradict the immunological recognition of the fetus or tumor as a separate entity, bridging the gaps between biology and philosophy and integrating the above ideas into one cohesive understanding. With both biological and philosophical perspectives, I will also erode the possibility of autonomy and agency for both the early-stage fetus and tumor both in isolation and within their hosts, as well as in medical and social contexts (Chigira et al., 1990; Kukla & Wayne, 2018). Finally, I will discuss the social and political implications of this argument as they pertain to abortion rights in a post-Roe world, especially in the restoration of autonomy and agency to the one true patient, the host.
Organizing committee:
- Lucie Laplane (IHPST & Gustave Roussy, Paris, France) (co-chair)
- Thomas Pradeu (ImmunoConcept, CNRS Bordeaux, France) (co-chair)
- Bertrand Daignan-Fornier (IBGC, CNRS Bordeaux, France)
- Benjamin Spada (ImmunoConcept, CNRS Bordeaux, France)
Scientific committee:
- Bertrand Daignan Fornier (CNRS Bordeaux, France)
- Andrew Ewald (Johns Hopkins, Baltimore, USA)
- Sara Green (University of Copenhagen, Denmark)
- Lucie Laplane (IHPST & Gustave Roussy, Paris, France)
- Maël Lemoine (University of Bordeaux, France)
- Carlo Maley (Arizona State University, USA)
- Violaine Moreau (CNRS Bordeaux, France)
- Julie Pannequin (CNRS, Institut de Génomique Fonctionnelle, Montpellier, France)
- Anya Plutynski (Washington University, USA)
- Thomas Pradeu (CNRS Bordeaux, France)
- Gregory Sepich-Poore (UCSD, USA)
- Vanja Sisirak (CNRS Bordeaux, France)
- Pierre Sujobert (University Claude Bernard, Lyon, France)
- Eric Solary (Gustave Roussy, Paris, France)
Funding
- This workshop is funded by the Gordon and Betty Moore Foundation through grant GBMF9021 to Thomas Pradeu (PI)
- The workshop is also supported by a small grant from ARC (Association pour la recherche sur le cancer) (1000 euros)
Registration (mandatory):
Location
– March 4th and 5th: Pessac Campus:
University of Bordeaux, Pessac Campus, Salle Manon Cormier
6 Av. Léon Duguit, 33600 Pessac, Map
– March 6th: La Victoire Campus (city center):
University of Bordeaux, La Victoire Campus, Amphitheater Sigalas
3ter Pl. de la Victoire, 33000 Bordeaux, Map
Call for abstracts (now closed)
The 6th Philosophy of Cancer Biology workshop will be held in Bordeaux, France, from March 4th to March 6th, 2024. Although invited talks will focus on the two topics of cancer microbiome and cancer metastasis, the call for abstracts is open to all emerging conceptual and theoretical issues in cancer research. Selected abstracts will have 15 minutes allocated for their talks followed by 15 minutes of discussion.
The conference is free and open to all researchers.
Young researchers can apply for a grant to help cover the cost of their travel and accommodation. Please contact Thomas Pradeu and Lucie Laplane
Abstracts must be submitted here ( https://forms.gle/CZo357M524Qy5mVu7 ) by Dec 5th. They will be anonymized and evaluated by the scientific committee. Notifications will be sent by December 22nd.
Submissions should contain:
– Title of talk
– Author(s). In case of multiple authors please indicate who will present.
– Institutional affiliation(s)
– Research field
– Position
– Abstract (no longer than 500 words). The abstract must identify a clear biological and/or medical problem and provide an answer to that problem using conceptual, theoretical, and/or philosophical tools. The scientific committee particularly welcomes interdisciplinary submissions as well as submissions that state explicitly what difference their proposal makes to scientific and medical practices.